The research on photocatalytic splitting of water and CO2 reduction for solar fuel production has been drawing great attention due to the increasing concerns on energy and environmental problems. These two reactions are energetically uphill and require multi-electron transfer, particularly the water splitting is regarded as the Holy Grail of science. One of the long-standing challenges in this filed is that most of photocatalysts possess suitable band structures and make the overall water splitting reaction thermodynamically possible, however, they cannot achieve overall water splitting actually.
Following this challenging issue, extensive research has been carried out by the Solar Energy Division (DNL 16) at DICP, led by Prof. Can Li. They have chosen different phases of titanium dioxide (TiO2), a popular and standard semiconductor used in photocatalysis, as examples to investigate the intrinsic reasons for photocatalytic overall water splitting. They found that the stable overall water splitting with stoichiometric H2/O2 ratio can be achieved on rutile TiO2. However, anatase and brookite TiO2 can only produce H2 at the initial stage, but H2 and O2 are obtained simultaneously on after long time irradiation. Further investigations by means of electron spin resonance spectra (EPR), transient infrared absorption-excitation energy scanning spectrum (TRIRA-ESS) and DFT calculations reveal that both thermodynamics and kinetics contributed to unique overall water splitting activity for different phases of TiO2. Kinetically the process of photocatalysis differs on different phases of TiO2 due to the intermediates (?OH radical for anatase and brookite TiO2, peroxy species for rutile TiO2) that are formed. Thermodynamically there are many trapped states lying below the Fermi levels of anatase and brookite TiO2 (but no trapped states can be found on rutile TiO2), which can reduce the overpotential for water oxidation. This finding will be helpful for understanding why many semiconductors are inactive as overall water splitting photocatalysts despite having thermodynamically suitable band structures for the proton reduction and water oxidation reactions. The work has been recently published in《Energy & Environmental Science》(Rengui Li, Yuxiang Weng and Can Li et al, Energy Environ. Sci., 2015, 8, 2377-2382.)
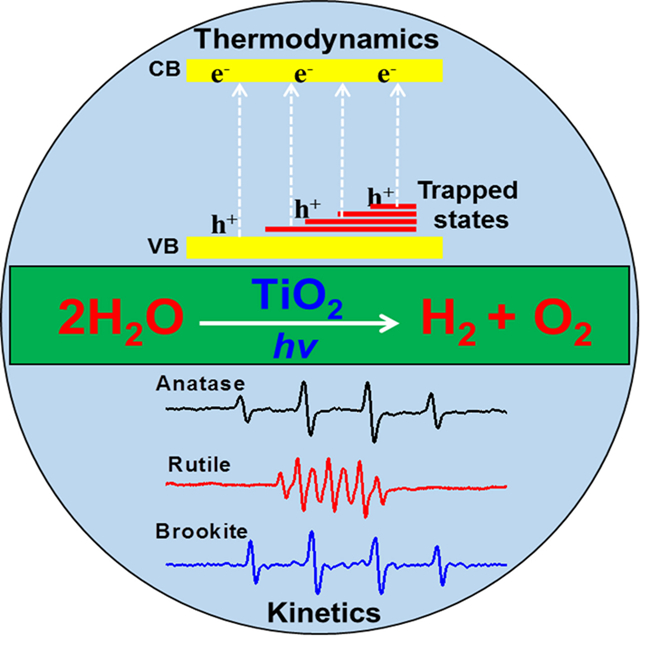
Our group Achieve Photocatalytic Overall Water Splitting on Titanium Dioxide-based Photocatalysts of Different Phases(Photo by Li Rengui)
This work was cooperated with Prof. Yuxiang Weng at Institute of Physics, CAS, and has been financially supported by NSFC and MOST 973 program. (Text/ Photo by Li Rengui)



