Recently,research group from Dalian Institute of Chemical Physics(DICP) and Dalian National Laboratory for Clean Energy (DNL) unraveled asingle-step two-electron transfer mechanism from photo-irradiated CdS tomolecular catalyst CoPy in CoPy/CdS hybrid photocatalytic systems under strongalkaline conditions. This work has been publishedrecently as a Communication inJournal of the American Chemical Society (J. Am. Chem. Soc., 2016, DOI: 10.1021/jacs.6b04080).
Thegroup has been working on the hybrid photocatalytic systems comprised ofsemiconductors and molecular catalysts for water reduction for many years (J. Catal.,2011, 281, 318; ChemSusChem, 2012, 5, 849; Chem. Commun., 2012, 48, 988; Acc. Chem.Res., 2013, 46, 2355; J. Catal., 2016, 338, 168).Itwas realized that the matching of the energy levels between semiconductorandmolecular catalyst is a crucial factor to be considered for the construction ofsuch hybrid systems. For most of the solar fuel production reactions,multi-electron transfer from light harvester to catalyst or reactant isnecessary. Therefore, better understanding the charge transfer mechanism(one-electron transfer or multi-electron transfer) between semiconductor andmolecular catalyst in photocatalytic system is important for the designing,synthesis and assembly of more efficient photocatalytic systems for watersplitting and CO2 reduction.
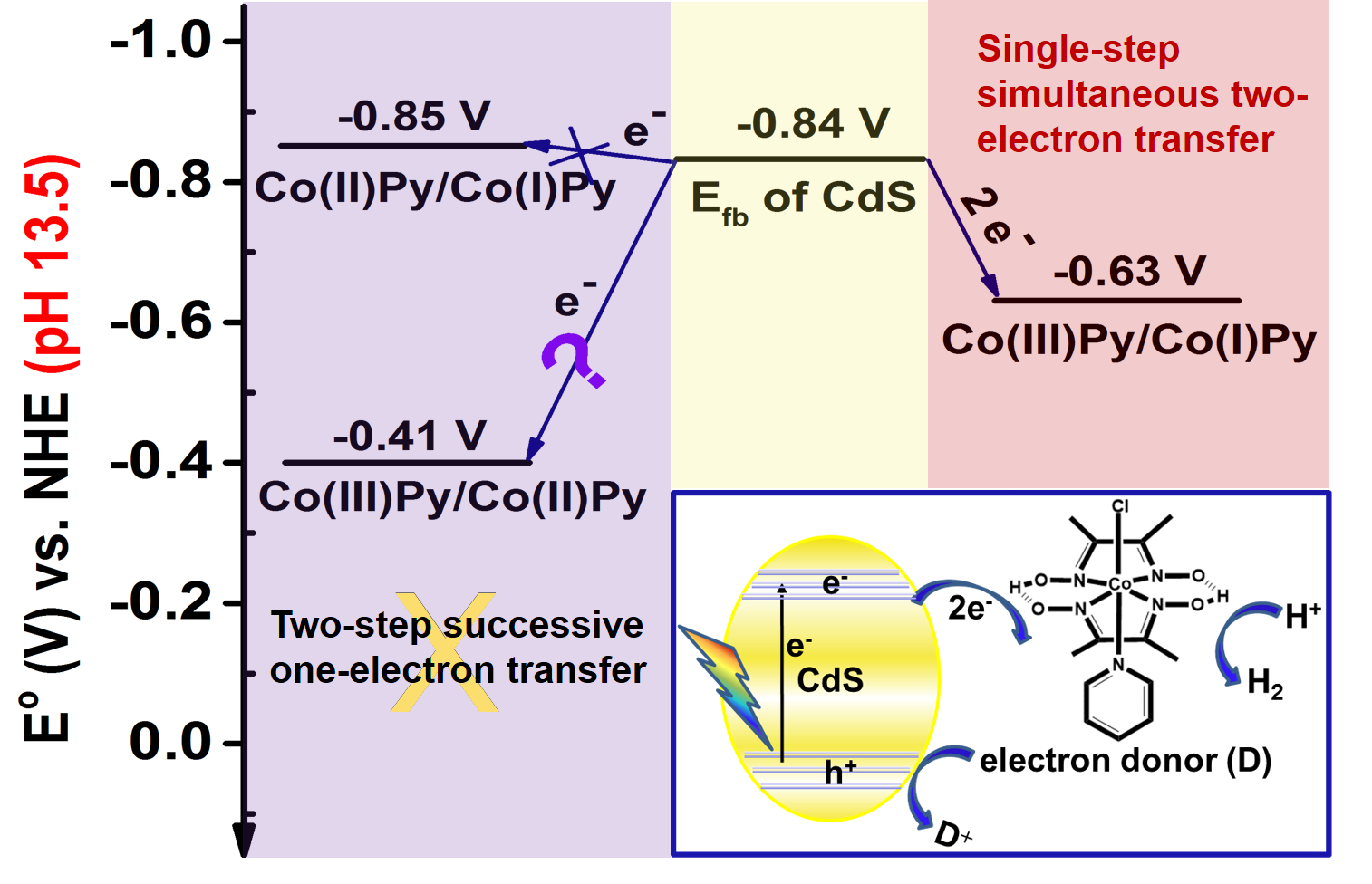
Inthis work, the electron transfer processes in Co(III)Py/CdS hybrid system underdifferent pH conditions have been investigated in detail. Energy level analysisindicates that two-step successive one-electron transfer from CdS to Co(III)Pyto yield the key proton reduction intermediate Co(I)Py species under high pHconditions (pH 13.5) is thermodynamically forbidden. However, enhancedphotocatalytic H2 evolution activity was indeed observed at pH 13.5.Charge transfer dynamics and kinetics studies showed that the single-step simultaneous two-electrontransfer from CdS to Co(III)Pyto yield the reduced Co(I)Py species isthe most plausible mechanism accounting for the enabling of the photocatalyticH2 evolution activity at pH 13.5.
Interms of the driving force, single-step simultaneous multi-electron transferprocess is energetically more favorable than successive multi-electron transferprocess according to Hess’s law. Therefore, revelation of single-stepsimultaneous two-electron transfer processes from semiconductor to molecularcatalyst is very important for the designing of more efficient semiconductor-molecularcatalyst hybrid system and optimization of the photocatalytic reactionconditions.
Thefirst author of this work is PhD candidate Yuxing Xu, co-supervised by Prof.Hongxian Han and Prof. Can Li. This work has been financially supported by NationalNatural Science Foundation of China, 973 National Basic Research Program of theMinistry of Science and Technology,and the Collaborative Innovation Center ofChemistry for Energy Materials. (Reported by Hongxian Han, Yuxing Xu).