In these years, our research on photoelectrocatalytic (PEC) water splitting has drawn worldwide attention. And the invited perspective “Photoelectrocatalytic Water Splitting: Significance of Cocatalysts, Electrolyte, and Interfaces” was recently published on ACS Catalysis. ( Chunmei Ding, Can Li*, et al., ACS Catal., 2017, 7, 675-688).
PEC water splitting is one of the ideal strategies of solar fuel production. And the solar-to-hydrogen (STH) efficiency is affected by many factors, and it is the production of the light absorption, charge separation, and charge injection efficiencies. To date, the efficiency of PEC water splitting is still far from the criterion for practical utilization, because many critical scientific issues are still unsolved, including the limited light absorption, sever charge recombination, high overpotential and slow kinetics of surface reactions, and poor stability of electrodes, etc. Since the group of Prof. Can Li initiated the research of PEC water splitting several years ago, great progress has been obtained on the fabrication of photoelectrodes (Nanoscale, 2014, 6, 2061; Phys. Chem. Chem. Phys., 2014, 16, 23544; Adv. Energy Mater. 2016, 1600864.), modification of cocatalysts (Phys. Chem. Chem. Phys., 2013, 15, 4589; ACS Appl. Mater. Interfaces 2015, 7, 3791; ChemSusChem 2015, 8, 3987.), effects of the electrolyte (J. Phys. Chem. B, 2015, 119, 3560.), charge storage and transport layers (Angew. Chem. Int. Ed. 2014, 53, 7295; Chem. Eur. J. 2015, 21, 9624; J. Phys. Chem. C 2015, 119, 19607; Energy Environ. Sci., 2016,9, 1327.), the electron-transport layer (ACS Appl. Mater. Interfaces, 2015, 7, 3791), manipulation of the interfacial energetics (J. Am. Chem. Soc., 2016, 138 (41), 13664.), construction of heterojunction (Chem. Sci. , 2016, 7, 6076.) and particle-particle interface charge transfer (Chem. Sci. , 2016, 7, 4391.), etc.
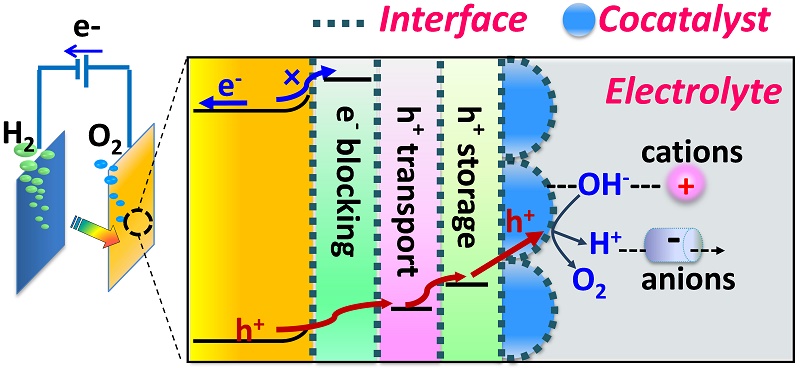
This Perspective describes recent progress on engineering electrode-electrolyte and semiconductor-cocatalyst interfaces with cocatalysts, electrolyte and interfacial layers (interlayers) to increase PEC efficiency. Key scientific issues about the function and effects of cocatalysts, interfacial layers and electrolyte ions on the separation and transfer of carriers, mechanisms of PEC water oxidation, and the effects of device design and other parameters during PEC water splitting will be discussed with some perspective views. Firstly, introducing cocatalysts has been demonstrated to be the most efficient way to lower the reaction barrier and promote the charge injection to reactants. In addition, electrolyte ions can influence the surface catalysis remarkably. Electrolyte cations on surface can influence the water splitting and backward reactions, and anions may take part in the proton transfer processes Moreover, the careful modification of the interface between cocatalysts and the semiconductor via suitable interlayers is critical for promoting the efficiencies of charge separation and transfer and the stability of the photoelectrodes.
This work was financially supported by the National Natural Science Foundation of China and 973 National Basic Research Program of the Ministry of Science and Technology of China. (Text and images/ Chunmei Ding and Can Li)